483 Document: Zevex Incorporated (dba MOOG Medical Devices Group), Sep 17, 2018
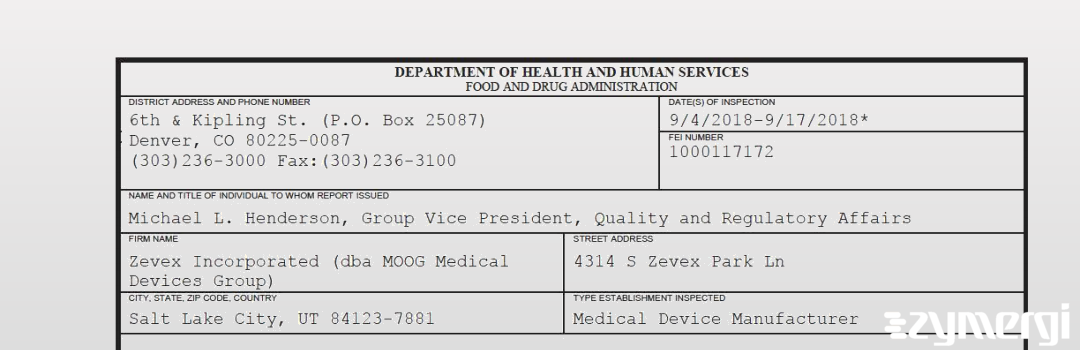

On Sep 17, 2018, the FDA inspected Zevex Incorporated (dba MOOG Medical Devices Group)'s Salt Lake City site. Explore the inspectional observations.


On Sep 17, 2018, the FDA inspected Zevex Incorporated (dba MOOG Medical Devices Group)'s Salt Lake City site. Explore the inspectional observations.