483 Document: Vygon MFG, Inc., dba/ Churchill Medical Systems, Inc., May 21, 2018
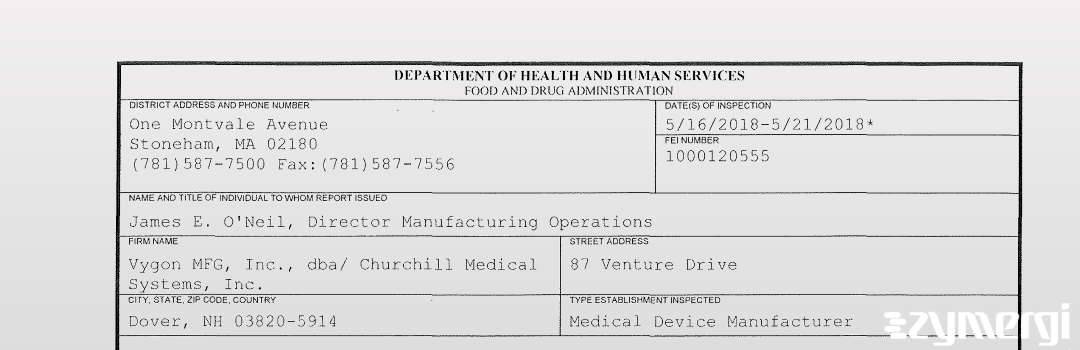

On May 21, 2018, the FDA inspected Vygon MFG, Inc., dba/ Churchill Medical Systems, Inc.'s Dover site. Explore the inspectional observations.


On May 21, 2018, the FDA inspected Vygon MFG, Inc., dba/ Churchill Medical Systems, Inc.'s Dover site. Explore the inspectional observations.