483 Document: Stryker Corporation, Nov 21, 2019
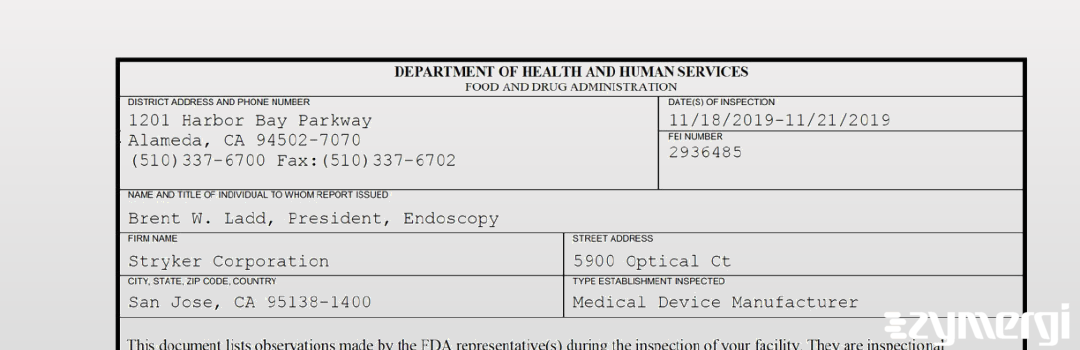
On Nov 21, 2019, the FDA inspected Stryker Corporation's San Jose site. Explore the inspectional observations.


On Nov 21, 2019, the FDA inspected Stryker Corporation's San Jose site. Explore the inspectional observations.