483 Document: SRI International, Aug 9, 2024
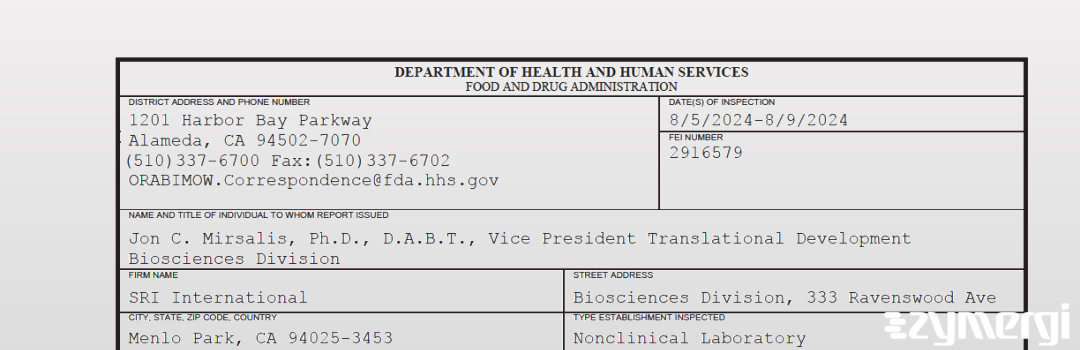
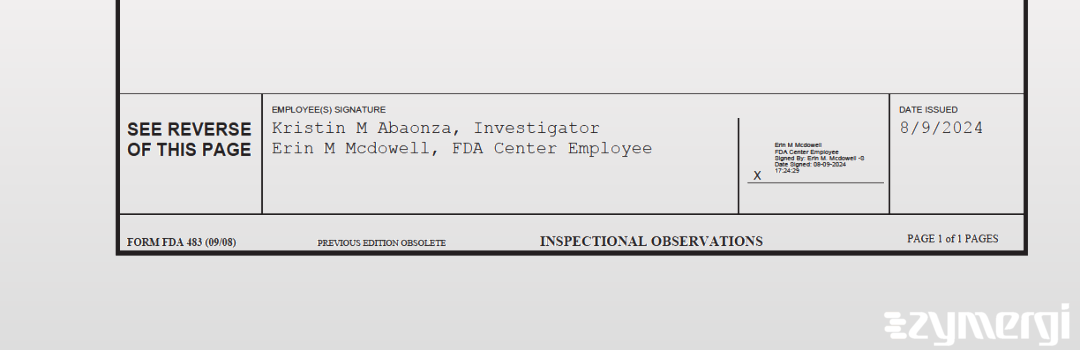
On Aug 09, 2024, the FDA inspected SRI International's Menlo Park site. Explore the inspectional observations.


On Aug 09, 2024, the FDA inspected SRI International's Menlo Park site. Explore the inspectional observations.