483 Document: Rudrajit Rai, M.D., Apr 10, 2019
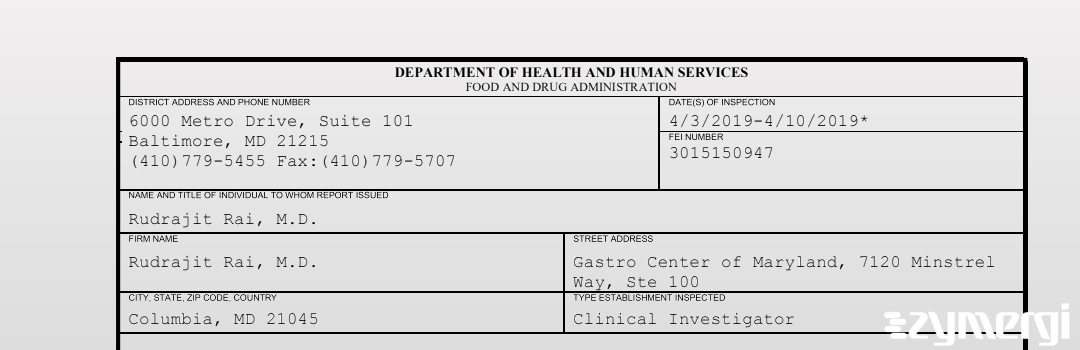

On Apr 10, 2019, the FDA inspected Rudrajit Rai, M.D.'s Columbia site. Explore the inspectional observations.


On Apr 10, 2019, the FDA inspected Rudrajit Rai, M.D.'s Columbia site. Explore the inspectional observations.