483 Document: McPherson Enterprises, Inc., dba Implantable Devices, Oct 29, 2021
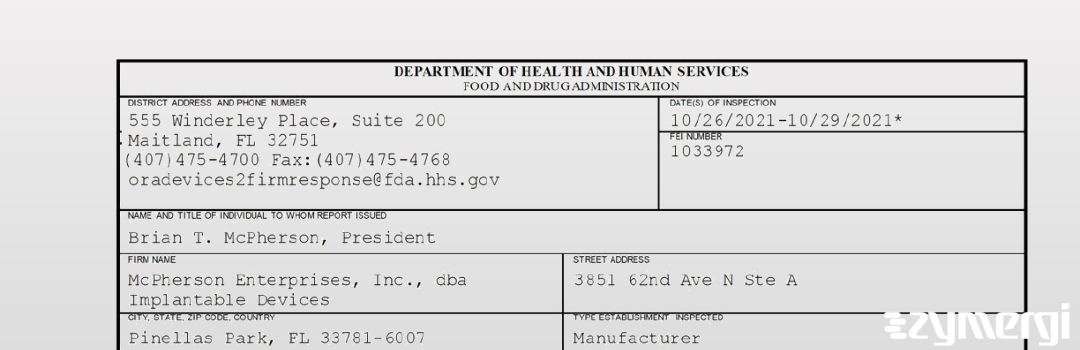

On Oct 29, 2021, the FDA inspected McPherson Enterprises, Inc., dba Implantable Devices's Pinellas Park site. Explore the inspectional observations.


On Oct 29, 2021, the FDA inspected McPherson Enterprises, Inc., dba Implantable Devices's Pinellas Park site. Explore the inspectional observations.