483 Document: M3 Global Enterprises LLC, Feb 16, 2023
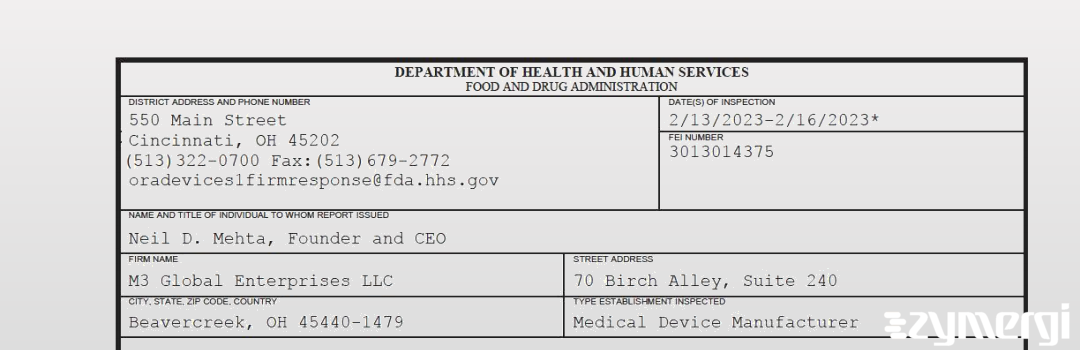
On Feb 16, 2023, the FDA inspected M3 Global Enterprises LLC's Beavercreek site. Explore the inspectional observations.


On Feb 16, 2023, the FDA inspected M3 Global Enterprises LLC's Beavercreek site. Explore the inspectional observations.