483 Document: Lupin Limited, Apr 4, 2022
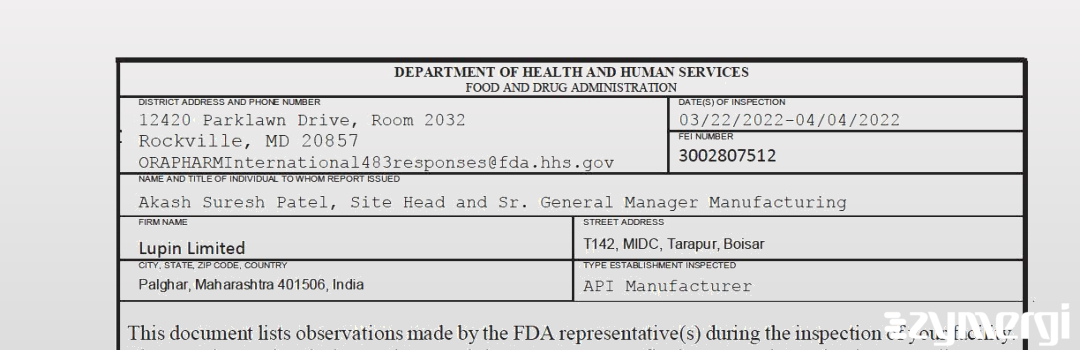

On Apr 04, 2022, the FDA inspected Lupin Limited's Tarapur, Thane site. Explore the inspectional observations.


On Apr 04, 2022, the FDA inspected Lupin Limited's Tarapur, Thane site. Explore the inspectional observations.