483 Document: Life Recovery Systems HD, LLC, Oct 21, 2019
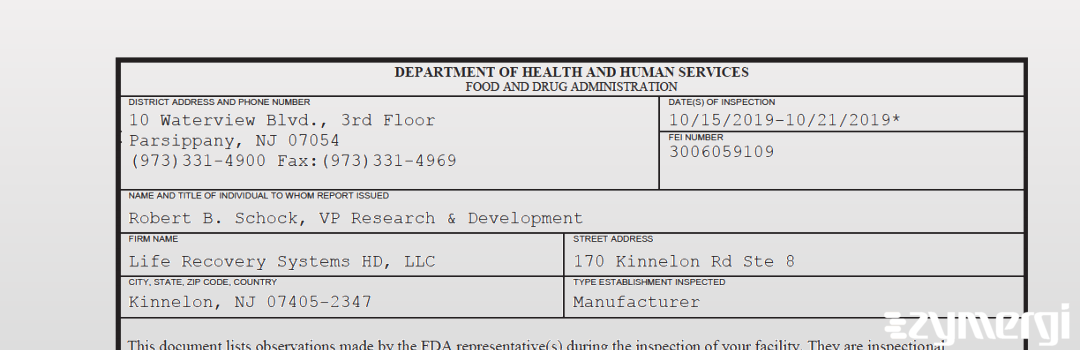

On Oct 21, 2019, the FDA inspected Life Recovery Systems HD, LLC's Kinnelon site. Explore the inspectional observations.


On Oct 21, 2019, the FDA inspected Life Recovery Systems HD, LLC's Kinnelon site. Explore the inspectional observations.