483 Document: KRS Global Biotechnology, Inc, Mar 17, 2014

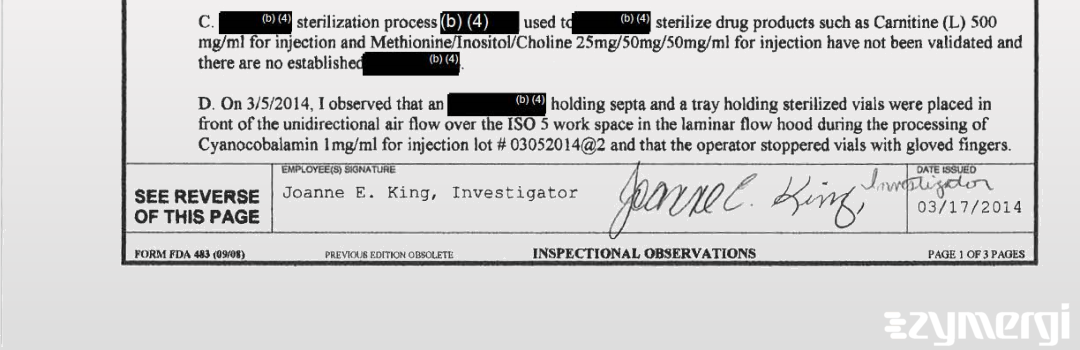
On Mar 17, 2014, the FDA inspected KRS Global Biotechnology, Inc's Boca Raton site. Explore the inspectional observations.


On Mar 17, 2014, the FDA inspected KRS Global Biotechnology, Inc's Boca Raton site. Explore the inspectional observations.