483 Document: Jubilant HollisterStier General Partnership, Jun 1, 2018
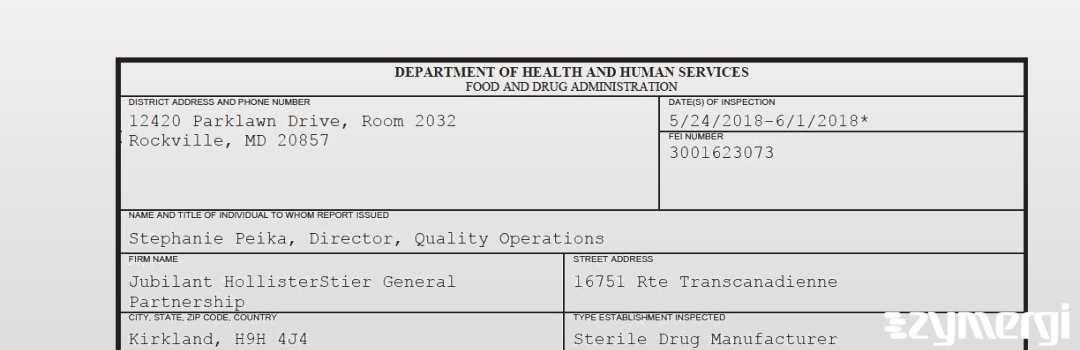
On Jun 01, 2018, the FDA inspected Jubilant HollisterStier General Partnership's Kirkland site. Explore the inspectional observations.


On Jun 01, 2018, the FDA inspected Jubilant HollisterStier General Partnership's Kirkland site. Explore the inspectional observations.