483 Document: JAS Diagnostics, Inc./Drew Scientific, Inc., Apr 28, 2022
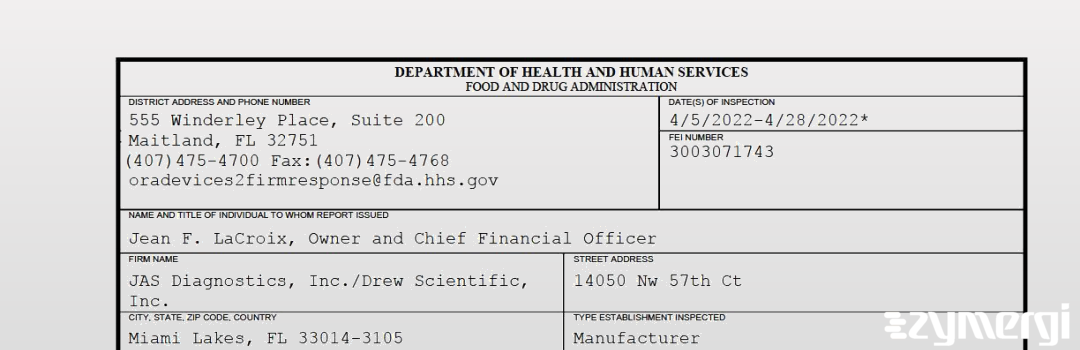

On Apr 28, 2022, the FDA inspected JAS Diagnostics, Inc./Drew Scientific, Inc.'s Miami Lakes site. Explore the inspectional observations.


On Apr 28, 2022, the FDA inspected JAS Diagnostics, Inc./Drew Scientific, Inc.'s Miami Lakes site. Explore the inspectional observations.