483 Document: IV Solutions of Lubbock, Mar 20, 2013
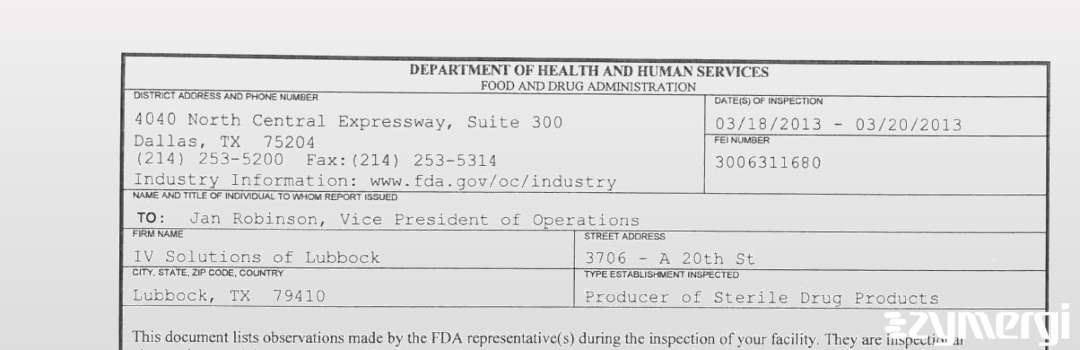

On Mar 20, 2013, the FDA inspected IV Solutions of Lubbock's Lubbock site. Explore the inspectional observations.


On Mar 20, 2013, the FDA inspected IV Solutions of Lubbock's Lubbock site. Explore the inspectional observations.