483 Document: Interpharm Praha A.S., Oct 16, 2015
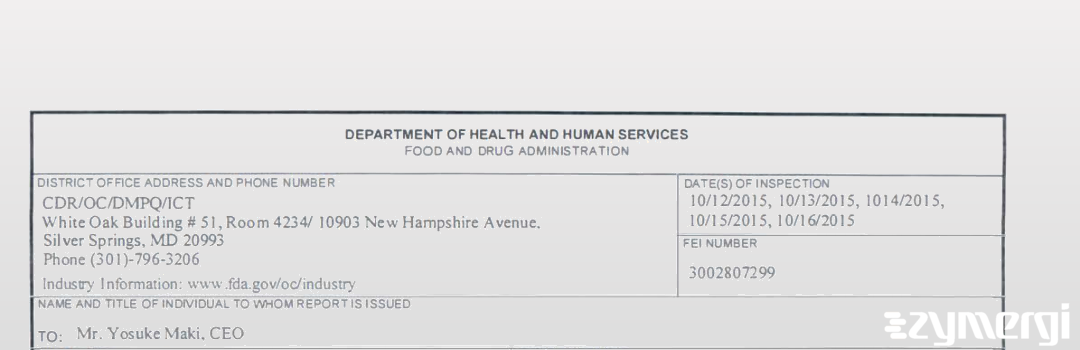

On Oct 16, 2015, the FDA inspected Interpharm Praha A.S.'s Prague 412 site. Explore the inspectional observations.


On Oct 16, 2015, the FDA inspected Interpharm Praha A.S.'s Prague 412 site. Explore the inspectional observations.