483 Document: Implant Direct Sybron Manufacturing LLC, Dec 2, 2019
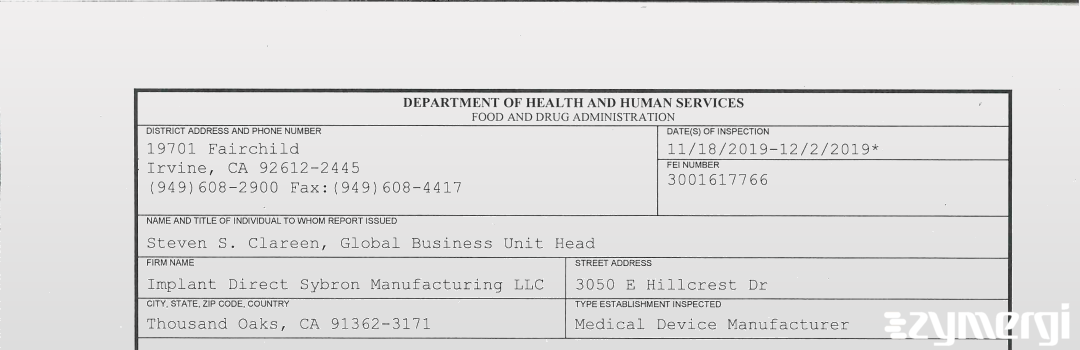

On Dec 02, 2019, the FDA inspected Implant Direct Sybron Manufacturing LLC's Westlake Village site. Explore the inspectional observations.


On Dec 02, 2019, the FDA inspected Implant Direct Sybron Manufacturing LLC's Westlake Village site. Explore the inspectional observations.