483 Document: GERI-GENTLE CORPORATION, Dec 4, 2017
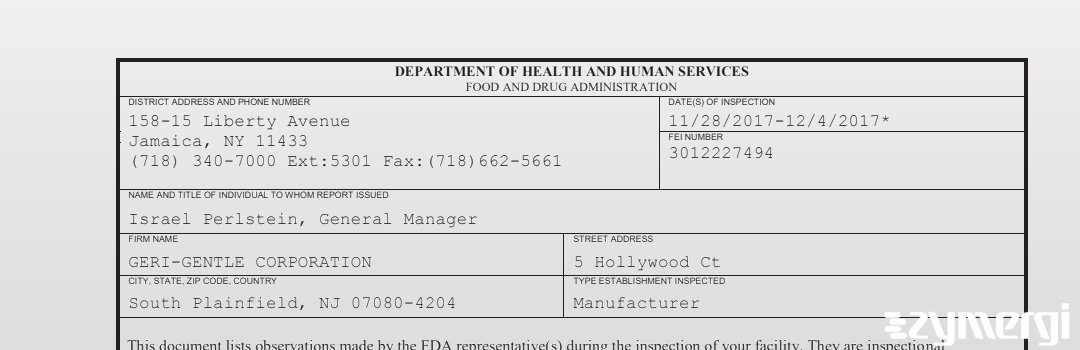
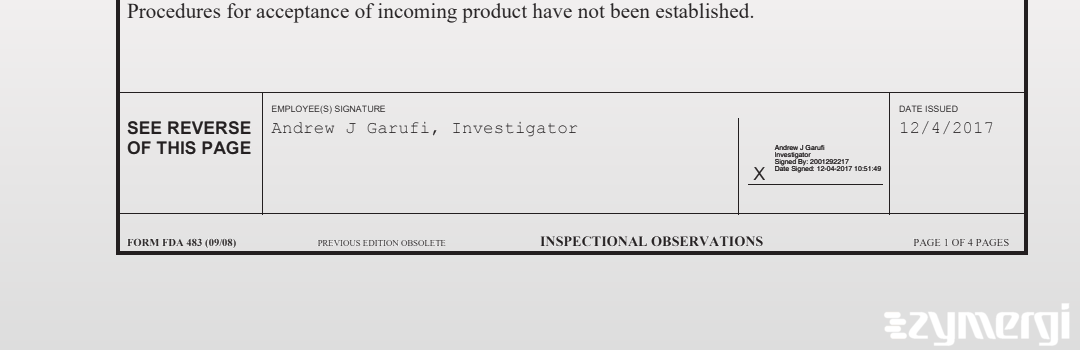
On Dec 04, 2017, the FDA inspected GERI-GENTLE CORPORATION's South Plainfield site. Explore the inspectional observations.


On Dec 04, 2017, the FDA inspected GERI-GENTLE CORPORATION's South Plainfield site. Explore the inspectional observations.