483 Document: Fusion IV Pharmaceuticals, Inc. dba Axia Pharmaceutical, Dec 20, 2019
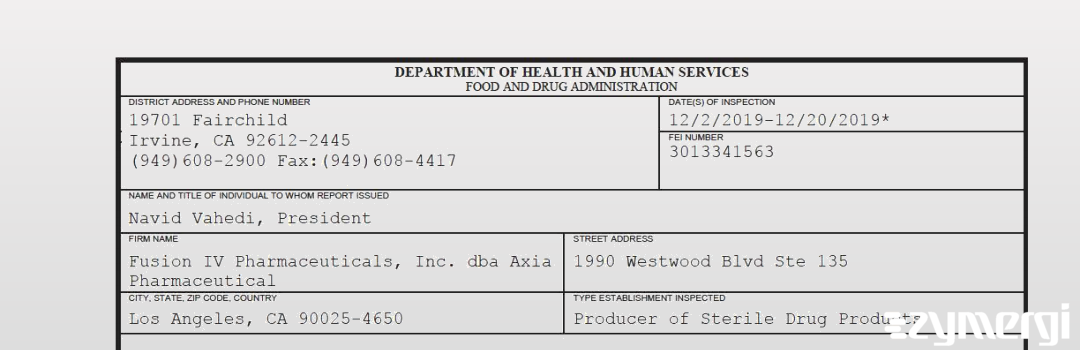

On Dec 20, 2019, the FDA inspected Fusion IV Pharmaceuticals, Inc. dba Axia Pharmaceutical's Los Angeles site. Explore the inspectional observations.


On Dec 20, 2019, the FDA inspected Fusion IV Pharmaceuticals, Inc. dba Axia Pharmaceutical's Los Angeles site. Explore the inspectional observations.