483 Document: IEC Electronics, Mar 11, 2022
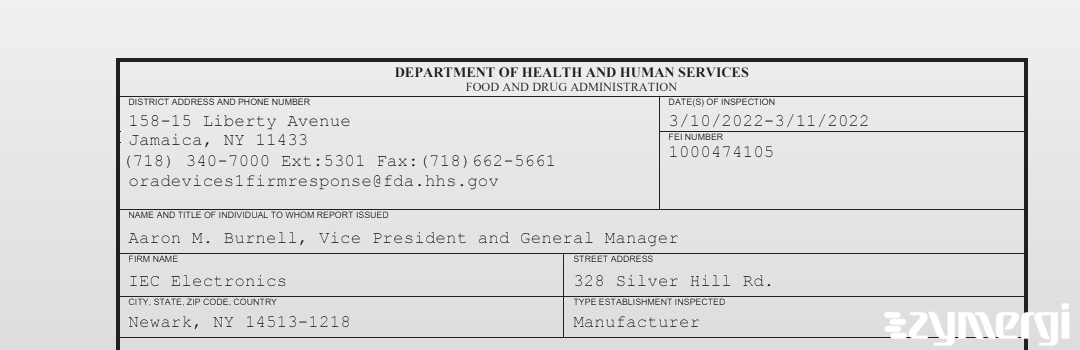

On Mar 11, 2022, the FDA inspected IEC Electronics's Newark site. Explore the inspectional observations.


On Mar 11, 2022, the FDA inspected IEC Electronics's Newark site. Explore the inspectional observations.