483 Document: Boehringer Ingelheim Pharma Gmbh & Co Kg, May 4, 2018
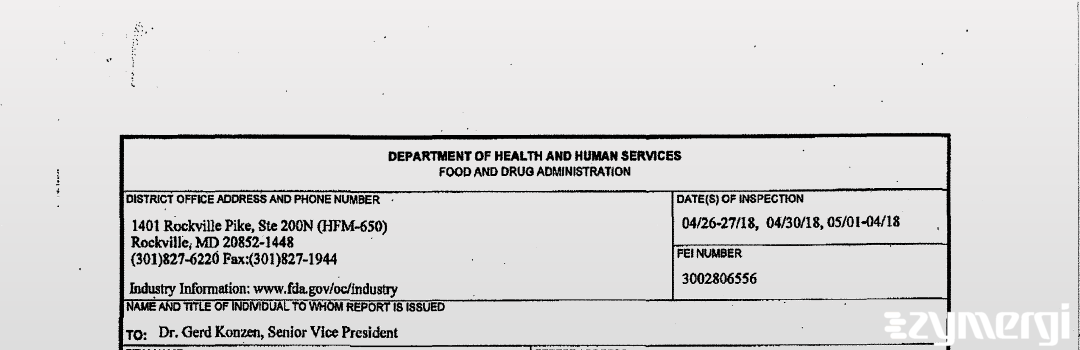

On May 04, 2018, the FDA inspected Boehringer Ingelheim Pharma Gmbh & Co Kg's Ingelheim am Rhein site. Explore the inspectional observations.


On May 04, 2018, the FDA inspected Boehringer Ingelheim Pharma Gmbh & Co Kg's Ingelheim am Rhein site. Explore the inspectional observations.