FDA Investigator: Claire M. Minden
- Claire M. Minden first started conducting FDA inspections in 2002, with the last inspection in 2022. Over that time, Claire M. Minden has conducted 421 inspections at 326 companies across 337 sites.
Get Claire Minden's official FDA inspection documents. Gain valuable insights from their Form 483s and EIRs to ace your next inspection.
Documents
Upon purchase, you will receive an email with a link to immediately download the documents.
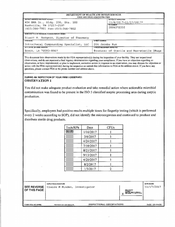
FDA 483 Takeda Pharmaceutical, Round Lake | September 2022
Available Now - $199
Claire M. Minden
Biologic Form 483
483 Response Patheon Manufacturing Services | Aug 2022
Available Now - $299
Claire M. Minden
Biologic Form 483
FDA EIR Patheon Manufacturing Services, Greenville | 2022
Available Now - $995
Claire M. Minden
Biologic Form 483
FDA 483 Catalent Maryland, Harmans | November 2021
Available Now - $199
Claire M. Minden
Biologic Form 483
FDA 483 CSL Behring L.L.C, Bradley | September 2021
Available Now - $199
Claire M. Minden
Biologic Form 483
FDA 483 Intrathecal Compounding Specialists, Scott | 2021
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Med Shop Total Care, Longview | July 2021
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Central Admixture Pharmacy Services | May 2021
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 PETNET Solutions, Covington | April 2021
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Blount Discount Pharmacy, Alcoa | February 2020
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 MPRX, . dba Medical Park Pharmacy, Dallas | Nov 2019
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Pharmaceuticals, Little Rock | November 2019
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Pharmacy Plus, . dba Vital Care Compounder | 2019
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Intrathecal Compounding Specialist, Scott | Feb 2019
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Med Shop Total Care, Longview | November 2018
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Cantrell Drug, Little Rock | August 2018
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Cardinal Health 414, New Orleans | August 2018
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Intrathecal Compounding Specialist, Scott | Nov 2017
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Pharmaceuticals, Little Rock | September 2017
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Coastal Meds, Biloxi | September 2015
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Total Pharmacy Services, Houma | September 2014
Available Now - $199
Claire M. Minden
Human Drug Form 483
FDA 483 Medistat RX L.L.C, Foley | September 2014
Available Now - $199
Claire M. Minden
Human Drug Form 483
483 Response Transdermal Therapeutics, Birmingham | Jun 2014
Available Now - $299
Claire M. Minden
Human Drug Form 483
FDA 483 Serum Institute of India Pvt. Ltd., Manjari | 2022
Out-of-stock
Claire M. Minden
Biologic Form 483
FDA 483 Evonik Corporation, Birmingham | 2020
Out-of-stock
Claire M. Minden
Human Drug Form 483
FDA 483 Ferring International Center S.A., St-Prex | 2019
Out-of-stock
Claire M. Minden
Human Drug Form 483
Co-Investigators (46)
- Thomas R. Withers, FDA Investigator
- Prabhu P. Raju, FDA Investigator
- Anissa M. Cheung, FDA Investigator
- Pankaj H. Amin, FDA Investigator
- Rajiv R. Srivastava, FDA Investigator
- Burnell M. Henry, FDA Investigator
- Debra M. Emerson, FDA Investigator
- Lakeesha M. Foster, FDA Investigator
- Tracy J. Washington, FDA Investigator
- Bonita S. Chester, FDA Investigator
- Nimmy Mathews, FDA Investigator
- Herbert M. Corbello, FDA Investigator
- Christopher N. Dedeaux, FDA Investigator
- Scott A. Watson, FDA Investigator
- Leisha R. Shipes, FDA Investigator
- Shannon Atlas, FDA Investigator
- Ivy E. Sweeney, FDA Investigator
- June P. Page, FDA Investigator
- Matthew R. McNew, FDA Investigator
- Ademola O. Daramola, FDA Investigator
- Wanda B. Coats, FDA Investigator
- Brandon C. Heitmeier, FDA Investigator
- Marvin D. Jones, FDA Investigator
- Cheryl L. Watson, FDA Investigator
- Wayne S. Fortenberry, FDA Investigator
- Konsuela Y. Glass, FDA Investigator
- Diana M. Guidry, FDA Investigator
- Kate E. Jesse, FDA Investigator
- Kip J. Hanks, FDA Investigator
- Chauncey A. Stephens, FDA Investigator
- Gloria J. Horner, FDA Investigator
- Daphne A. Videau, FDA Investigator
- Traci M. Armand, FDA Investigator
- Phuong L. Tran, FDA Investigator
- David R. Heiar, FDA Investigator
- Barbara D. Wright, FDA Investigator
- Mark W. Rivero, FDA Investigator
- Wendy R. Blame, FDA Investigator
- Natalie A. Guidry, FDA Investigator
- Elizabeth D. Connell, FDA Investigator
- Verlinda A. Narcisse, FDA Investigator
- Lakisha N. Morton, FDA Investigator
- Dawn P. Hall, FDA Investigator
- Toni M. Booker, FDA Investigator
- Margaret E. Slimbach, FDA Investigator
- Sidney M. Smith, FDA Investigator