483 Document: Nova Southeastern University IRB #1, Aug 17, 2023
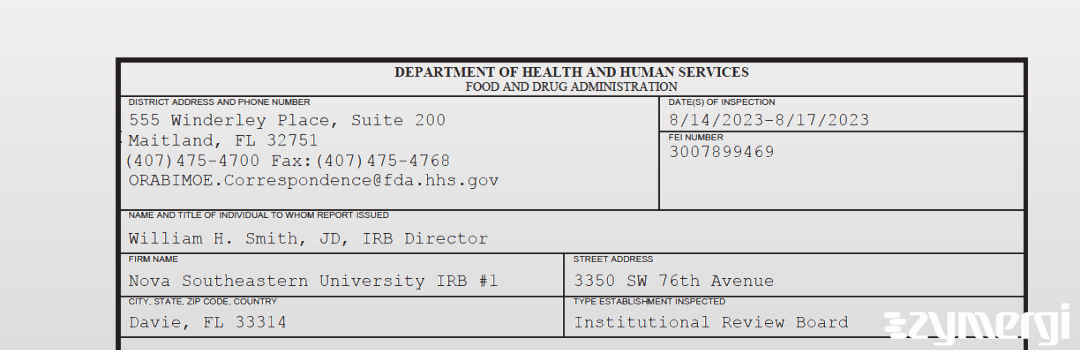
On Aug 17, 2023, the FDA inspected Nova Southeastern University IRB #1's Davie site. Explore the inspectional observations.


On Aug 17, 2023, the FDA inspected Nova Southeastern University IRB #1's Davie site. Explore the inspectional observations.