483 Document: Kohana Pharmacy and Center for Regenerative Medicine Inc., Apr 17, 2019
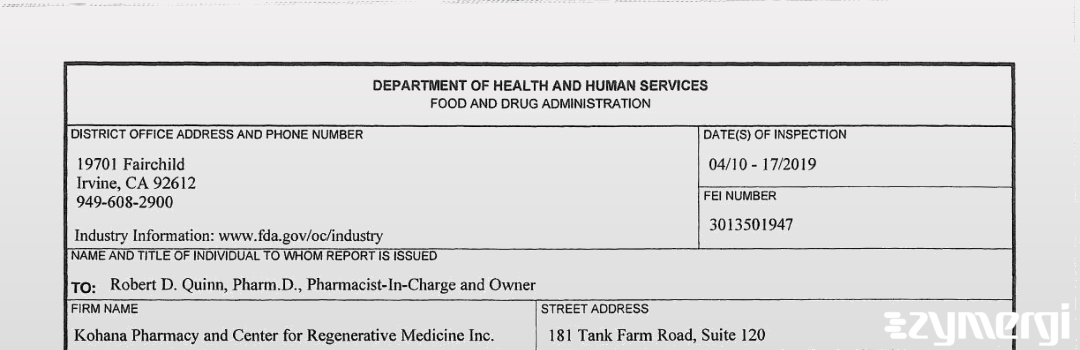
On Apr 17, 2019, the FDA inspected Kohana Pharmacy and Center for Regenerative Medicine Inc.'s San Luis Obispo site. Explore the inspectional observations.


On Apr 17, 2019, the FDA inspected Kohana Pharmacy and Center for Regenerative Medicine Inc.'s San Luis Obispo site. Explore the inspectional observations.