483 Document: 86 Harriet Ave Corporation DBA General Devices, Jun 11, 2015

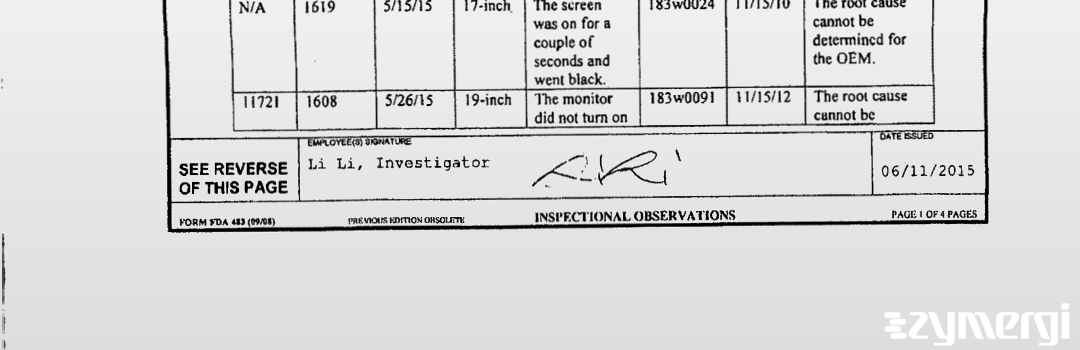
On Jun 11, 2015, the FDA inspected 86 Harriet Ave Corporation DBA General Devices's Ridgefield site. Explore the inspectional observations.


On Jun 11, 2015, the FDA inspected 86 Harriet Ave Corporation DBA General Devices's Ridgefield site. Explore the inspectional observations.